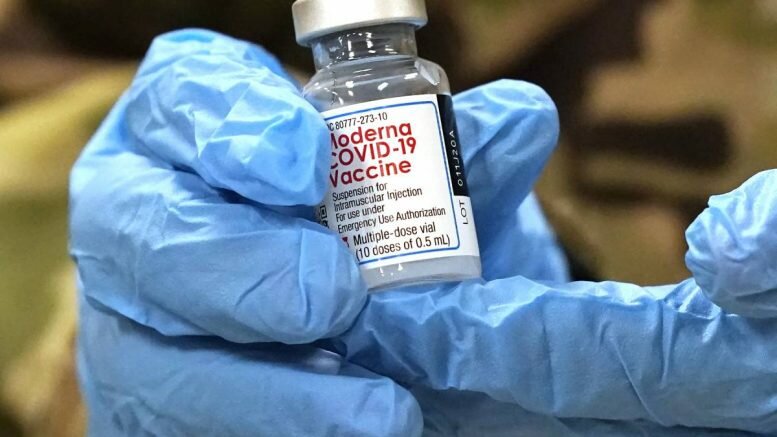
In a few days, Norway will be receiving the first shipment of the coronavirus vaccine from Moderna. It is very similar to the Pfizer vaccine, but the related logistics and distribution could be simpler.
Moderna’s COVID-19 vaccine was approved for use in the EU and Norway on Wednesday.
“The coronavirus vaccines from Pfizer-BioNTech and Moderna are both based on RNA technology. The vaccines are very similar,” chief physician Sigurd Hortemo at the Norwegian Medicines Agency noted.
“They are based on the same principle, with some differences at the level of detail.
“Both contain a recipe that the body’s cells can use to mark the characteristic “nail” on the COVID-19 virus.
“The body perceives the nail as something foreign, and we get an immune response,” Hortemo explained.
Longer shelf life
One of the big differences between the vaccines is the logistic part and the distribution to municipalities and nursing homes.
“The difference is greatest in practical terms since the Moderna vaccine can be stored in a regular freezer,” Hortemo added.
Storage at minus 20 degrees is considered a major advantage of the Moderna vaccine, while the Pfizer vaccine requires storage at minus 70 degrees.
Furthermore, its shelf life is also longer in the refrigerator after thawing.
The Pfizer vaccine must be used within five days, while the Moderna vaccine can be refrigerated for 30 days and can also be stored at room temperature for up to twelve hours.
Small differences
People who are to be vaccinated will probably not notice much difference between the two vaccines.
Both require two doses: the refresher dose is administered three weeks (21 days) after the first one for the Pfizer vaccine and four weeks (28 days) for Moderna vaccine.
Another difference is that Moderna has chosen a more substantial dose in its vaccine (100 micrograms) than Pfizer (30 micrograms).
Both companies have good testing results. Pfizer-BioNTech was able to report a 95% effective vaccine one week after the second dose.
There should also not have been much difference in effect in different age groups or ethnic groups.
The Moderna vaccine is close, with 94.1% efficacy. The company has made a reservation that the final results are not ready and that the level may change somewhat.
The vaccines have not been tested on children and adolescents, pregnant or breastfeeding women.
Side effects
Like Pfizer’s vaccine, Moderna’s vaccine was tested on a large group of people before the company applied for approval.
A total of 30,000 people participated in the test period, where half received a placebo vaccine, and the other half received the real vaccine.
The vaccine is already in use in the United States and the United Kingdom.
There is a relatively large proportion of the vaccinated who experience local side effects after a few days, the manufacturers state.
So far, there is nothing special that separates the two vaccines when it comes to the side effects.
Side effects can come in the form of pain at the injection site and more general side effects such as fatigue, headaches, muscle aches, chills, and joint pain.
The symptoms are mild to moderate and go away after a few days.
However, the side effects can be so unpleasant that some people feel that they cannot go to work.
Therefore, the National Institute of Public Health (FHI) recommends that this fact be taken into account when health personnel is vaccinated.
Norway has secured 1.9 million doses of the Moderna vaccine, but it has been reported that it will receive only 67,000 doses until the end of February.
© NTB Scanpix / #Norway Today / #NorwayTodayNews



Be the first to comment on "Norwegian Medicines Agency: Pfizer’s and Moderna’s corona vaccines are very similar"