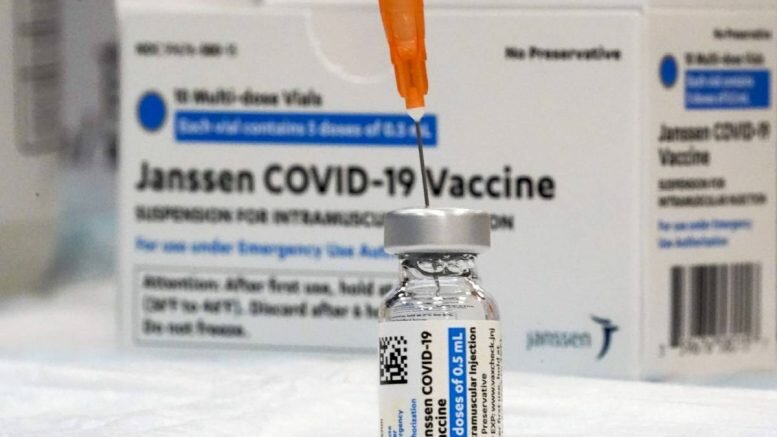
Cases of people in the USA experiencing blood clots after receiving Janssen’s COVID-19 vaccine have led to the EU Medicines Agency opening an investigation into the issue.
The cases to be examined are of the same type as those associated with the AstraZeneca vaccine – blood clots in combination with a low platelet count.
The EMA stated on Friday that they had established a so-called signal case and that the vaccine will be examined. The case was first reported in Norway by Norwegian Broadcasting (NRK).
In total, there are allegedly four cases of blood clots in the United States. More than 4.8 million people in the United States have received the vaccine, also known as the Johnson & Johnson vaccine.
The vaccine stands out in that only one dose is needed to be fully vaccinated.
Approved in the EU and Norway
One of the American cases occurred in the last phase of the vaccine testing, while the other three cases are newer. One of the patients died, according to the EMA.
So far, the Janssen vaccine has only been used in the United States, but it has also been approved in the EU and Norway. It is scheduled to be used in Europe within a few weeks.
The Norwegian authorities expect to receive a large number of doses of the vaccine in June, and it may therefore be of great importance for the vaccination of younger people.
“Four serious cases of blood clots have been reported after using the vaccine. The Norwegian Institute of Public Health believes it is good that this is taken seriously and that the EMA investigates the issue,” the FHI director of infection control Geir Bukholm said in a statement to NTB.
No causal link established so far
Bukholm emphasized that, so far, no causal link has been established between the Janssen vaccine and the blood clots.
“We will look at this more in our assessments of the use of the vaccine in Norway,” he said about the EMA’s investigation.
Steinar Madsen, medical director at the Norwegian Medicines Agency, told NRK that these may be very rare side effects.
Already in mid-March, the Medical Products Agency in Sweden stated that they were monitoring whether the Janssen vaccine could increase the risk of blood clots, based on a study of the vaccine.
Source: © NTB Scanpix / #Norway Today / #NorwayTodayNews
Do you have a news tip for Norway Today? We want to hear it. Get in touch at info@norwaytoday.no


Be the first to comment on "EU starts investigating blood clot cases among people vaccinated with the Johnson & Johnson vaccine"